Actovegin review 2023: Benefits, side effects, Actovegin sports scandal, dosages & FAQ
July 28, 2023
Table of contents
- What is Actovegin?
- Actovegin Takeda manufacturer
- Who uses Actovegin?
- What is Actovegin used for?
- What are Actovegin effects?
- What are Actovegin benefits?
- Actovegin research
- What are Actovegin side effects?
- Actovegin use in pregnancy. Actovegin contraindications
- Actovegin vs Solcoseryl
- Actovegin sports use
- Actovegin doping scandal
- Is Actovegin safe?
- Anecdotal Actovegin Reddit reviews
- Where to buy Actovegin?
- What is Actovegin dosage?
- Actovegin injections vs Actovegin pills, which one is better?
- Conclusion
- Actovegin review bibliography
What is Actovegin?
Actovegin is a widely used antihypoxic nootropic that helps to increase oxygen supply to cells and improve energy metabolism. In essence, Actovegin is a deproteinized calf blood extract obtained by ultrafiltration.
This nootropic is made up of more than 200 components including physiological amino acids, oligopeptides, nucleosides, intermediates of carbohydrate and fat metabolism, antioxidant enzymes, electrolytes, and trace elements. There is no precise data on Actovegin pharmacokinetics, as it is a multicomponent drug and its composition includes substances originally present in the human body.
Although the exact Actovegin mechanism of action is not yet fully understood, studies have already established a number of effects of the mixture:
- metabolic
- neuroprotective
- microcirculatory
Actovegin uses include the treatment of vascular and metabolic disorders of the brain, circulatory disorders, and their consequences (trophic ulcers), burns and wounds, and fetal growth disorders in pregnant women.
Actovegin Takeda manufacturer
The nootropic complex is manufactured by the Japanese company Takeda Pharmaceutical which has operated since 1781. It is one of the leaders in healthcare worldwide.

Who uses Actovegin?
Among the first users of Actovegin Takeda were athletes, and cyclists. Then sportsmen began to find applications of Actovegin in bodybuilding. They mostly use Actovegin in case of muscle sports injuries.
Actovegin is also commonly used by patients with cerebrovascular disorders and by the elderly who suffer from mild cognitive disorders prior to dementia.
What is Actovegin used for?
Actovegin is recommended by doctors for widespread use. For example, in the form of Actovegin ointment, the substance is used in the treatment of dermatologic pathology due to its ability to accelerate tissue healing.
Meanwhile, the main forms of administration are Actovegin solution and Actovegin pills. And neurological diseases are the main indication for its use. As part of complex therapy, it can be applied in the following cases:
- Symptomatic treatment of cognitive disorders, including post-stroke cognitive disorders and dementia.
- Symptomatic treatment of peripheral circulation disorders and their consequences.
- Symptomatic treatment of diabetic polyneuropathy (DPN).
What are Actovegin effects?
Antihypoxic action
In diseases accompanied by ischemia and hypoxia, oxygen and glucose deficiency develop, resulting in impaired synthesis of adenosine triphosphate (ATP) and decreased cellular energy reserves.
Experimental studies have shown that Actovegin increases oxygen uptake and utilization and, as a consequence, improves energy metabolism and cell resistance to hypoxia [7]. As a result of the normalization of oxygen and glucose supply to tissues, the formation of macroergic phosphates (ATP, ADP) increases and energy cellular imbalance decreases. Enhancement of oxygen absorption by the vascular wall during Actovegin administration results in the normalization of endothelium-dependent reactions and reduction of peripheral vascular resistance.
In addition, Actovegin activates glucose transporters GLUT1 and GLUT4, which, for example, in cerebrovascular diseases may enhance glucose transport across the blood-brain barrier [13]. The presence of such a mechanism was confirmed in a clinical study by D. Ziegler et al. (2009), in which Actovegin administration in patients with polyneuropathy against the background of type II diabetes mellitus significantly reduced the level of glycosylated hemoglobin (HbA1C) compared to placebo [10].
Due to the fact that Actovegin modulates the activity of intracellular glucose transport [3], lipolysis is activated. The possibility of using Actovegin for the treatment of diabetes mellitus and metabolic syndrome is under consideration. [6, 9]
Neuroprotective effects
Recently, several studies have been conducted to investigate the neuroprotective effects of Actovegin and its ability to enhance the survival of neurons.
An in vitro study conducted in cultivated primary rat hippocampal neurons demonstrated the neuroprotective and regenerative properties of Actovegin [11]. Normally, the number of cultivated neurons decreases dramatically as a result of apoptosis. However, after 10 days in the culture under optimal conditions, an Actovegin-dose-dependent increase in the number of viable neurons (2.4 times higher compared to the control) was observed.
Similar effects are noted in the peripheral nervous system of rats with severe symptoms of diabetic neuropathy [14]. Administration of Actovegin at a dose equivalent to the clinical dosage for humans resulted in a decrease in peripheral neuronal degeneration caused by diabetes mellitus. At the end of the study, Actovegin increased intraepidermal nerve fiber (IENF) density by 32% and restored the reduced rate of nerve impulse conduction in sensitive fiber by up to 91%.
Antioxidant effect
Actovegin antioxidant effect is achieved by the presence of superoxide dismutase and magnesium ions in the composition of the drug, which increase the activity of glutathione synthetase, which converts glutathione into glutamine.
It is shown that Actovegin increases the rate of redox processes in hepatocytes, reduces ultrastructural and functional damage to mitochondria of cardiomyocytes, and increases a reduced level of glucose metabolism in chronic alcoholism.
What are Actovegin benefits?
Actovegin benefits include the ability to improve glucose absorption, cellular metabolism, and respiration in tissue. This results in the activation of regeneration processes.
Ultimately Actovegin nootropic can enhance physical performance and stamina as well as have a calming effect on the nervous system.
Actovegin research
Actovegin cognitive effect
In a study of Actovegin effect on age-related memory impairment, it was revealed that after two weeks of drug therapy, a statistically significant improvement in regard to attention, memory, and thinking processes was observed. Moreover, even a single administration of the drug led to an improvement in electrophysiological indices of brain function [1, 2].
A study involving 120 patients with cerebrovascular insufficiency and cognitive deficit showed that in long-term therapy of dyscirculatory encephalopathy with the syndrome of cognitive impairment preference should be given to oral administration of Actovegin [4].
Actovegin and diabetes
The possibility of using Actovegin, given its effect on glucose utilization, in type 2 diabetes mellitus (DM) patients with diabetic encephalopathy for the treatment of cognitive impairment is of great interest.
In a study of 60 patients with type 2 DM who had cognitive impairment of various degrees of severity, Actovegin intravenous administration at a dose of 400 mg for three weeks led to an improvement in the sum of scores on the MMSE scale, with the greatest improvement being in memory [8]. It can be assumed that the clinical efficacy of Actovegin in type 2 DM patients with cognitive impairment is primarily due to an improvement in cerebral metabolism.
In 2009, the American scientific journal “Diabetes Care” published a study evaluating the efficacy and safety of Actovegin for the treatment of patients with diabetic polyneuropathy. The RCT involved 567 patients with type 2 diabetes mellitus. The study concluded that sequential intravenous and oral Actovegin treatment over 160 days improved neuropathic symptoms, VPT, sensory function, and quality of life in type 2 diabetic patients with symptomatic polyneuropathy. [10]
W. Jansen and E. Beck studied the effect of Actovegin in patients with diabetic polyneuropathy in a controlled trial. One group of 35 patients received placebo, another group of 35 patients received Actovegin at a dose of 600 mg 3 times a day for 24 weeks [5]. Improvement of the patients’ condition in the Actovegin treatment group was noted in the majority of patients 8 weeks after the start of treatment, and the optimal effect was achieved after 16 weeks of treatment. The reliable improvement compared to the placebo group was shown in almost all clinical parameters: walking distance without pain, tendon reflexes, surface, and deep sensitivity. Patients in the Actovegin group felt better and had fewer complaints of psycho-emotional disturbances, which correlated with the improvement in their physical condition.
Actovegin after stroke
In 2017, a large-scale international prospective randomized placebo-controlled study of Actovegin was conducted, which involved more than 500 stroke patients. The effect of the drug on the recovery process after ischemic stroke was studied (in particular, the ADAS-cog+ Alzheimer’s Disease Cognitive Assessment Scale and the Montreal Cognitive Assessment Scale MoCA were measured). The study lasted 12 months, with scores measured at months three, six, and twelve. In the third month, no statistically significant difference was achieved between the placebo and Actovegin groups (it amounted to 1.1 points on the ADAS-cog+ scale). By the sixth month, the difference on the same index was 2.3 points and was considered statistically significant. The treatment was discontinued. By the 12th month, the difference was 3.7 points. The conclusion of the study states that Actovegin had a positive effect on cognitive impairment in stroke patients. Additionally, more rigorous controlled studies are needed to confirm this result. [16]
One of the most recent international randomized placebo-controlled trials was conducted between 2018 and 2020. The study enrolled 366 patients with Fontaine stage IIB peripheral arterial disease (PAD). The primary studied metric was the percent change in pain-free walking distance (ICD) from baseline at the 12th week. Based on the results, Actovegin showed statistically significant superiority over placebo. Actovegin also demonstrated a level of safety comparable to the placebo group [17].
What are Actovegin side effects?
Regardless of the dosage form, the most common side effects include skin hyperemia, and rash. An anaphylactic reaction is possible in case of hypersensitivity.
Actovegin use in pregnancy. Actovegin contraindications
There are studies saying that Actovegin can be beneficial in case of the growth retardation of the fetus. [15] However, the official Actovegin instructions clearly indicate that treatment with the drug in pregnancy is permissible only if the benefit exceeds the potential risk (!) to the fetus.
Other Actovegin contraindications include:
- Hypersensitivity,
- Fructose intolerance, glucose-galactose malabsorption or sugar-isomaltase deficiency,
- Children under 18 years old.
Actovegin 5 vs Solcoseryl
Actovegin originated as a generic of another drug – Solcoseryl that has been produced since 1996 by the Swiss company Solco.
The active ingredient of both preparations is deproteinized hemodialysate of calf blood, i.e. blood devoid of proteins and other particles larger than 5 kilodaltons. Solcoseryl is available as an ointment, while its generic is also available in the form of Actovegin solution for injection and Actovegin tablets.
Actovegin is an antihypoxic compound with metabolic, neuroprotective, and microcirculatory effects. It is used in the therapy of diseases of the peripheral circulatory system and in the treatment of cognitive disorders, including dementia and post-stroke. A distinctive indication is the treatment of diabetic polyneuropathy.
Solcoseryl is used in combination therapy in disorders of metabolism and blood circulation in the brain (ischemic and hemorrhagic stroke, craniocerebral trauma) and pathologies of peripheral vessels. Solcoseryl is not prescribed during pregnancy.
In effect, the drugs are almost complete analogs. Because of the common origin and similar indications, it is difficult to give an advantage to one of them. Only the attending physician can decide which one is better, Actovegin or Solcoseryl.
Actovegin sports use
Actovegin is considered an ergogenic drug, although there is no evidence of its stimulatory effect on athletic performance. One of the hypothesized mechanisms of action of this drug is claimed to be an increase in tissue oxygen levels. Athletes take Actovegin, bodybuilding included, in order to improve their physical capabilities and to achieve:
- Faster metabolic reactions within cells;
- Reduced production and accumulation of lactic acid in tissues;
- Improved contractility of muscle fibers;
- Increased energy reserves in working muscles;
- Accelerated supply of more oxygen to the cells;
- Improved blood flow to the muscles;
- Increased glucose transport rate.
How are athletes taking Actovegin?
Before taking Actovegin in sports, it is necessary to determine your goal. Thus, for accelerated recovery while preparing for competitions (as well as during and after them) it is possible to use such schemes: Actovegin 200 mg pills*3 times/day; or Actovegin intramuscular injections 5 ml*2 times/day.
In conditions of hypoxia, the Actovegin drug is administered 80 mg parenterally for 14 days or in tablet form 200 mg*3 times/day for 2 to 6 weeks.
Before the first parenteral administration of the product, it is necessary to make a test, as it can cause allergies.
The most frequently recommended dosage is the Actovegin injection for athletic use, which is considered to be more effective compared with the Actovegin pill form.
Actovegin doping scandal
Lance Armstrong, calf-blood extract in Tour de France
In 2000, Actovegin was at the center of a sports scandal. Participants of the Tour de France cycling race, including Lance Armstrong, its seven-time winner, were accused of using it. Despite the fact that it is difficult to detect traces of the drug in the blood (our own blood contains approximately the same substances), the grounds for the accusation were open packages of Actovegin that were found during the inspection. However, as further research showed, the drug was deemed not effective enough. The charges were dropped.

Brandt-Sorenson pleaded guilty to selling performance-enhancing Actovegin
In 2016, the former cycling athlete Brandt-Sorenson pleaded guilty because he sold Actovegin to his fellow athletes, along with other performance-enhancing substances. Nick Brandt-Sorenson accepted a lifetime ban for his doping offenses. Brandt-Sorenson is now retired from competition. He is engaged in designs of made-to-measure premium cycling apparel.
As of the writing of this article, Actovegin is excluded from the list of doping by WADA. And athletes continue to widely use it in various sports to improve performance and for rapid recovery.
Is Actovegin safe?
Some may wonder why Actovegin is not approved by the FDA for medical use in the US and Canada. Possible reasons for such a restriction are believed to be its unproven efficacy and the spreading danger of infection with prion diseases that affect the nervous system of mammals. In cows, it is spongiform encephalopathy (aka mad cow disease), and the human version is called Creutzfeldt-Jakob disease. Prion diseases are caused by a misfolded protein that “infects” other proteins with its form, leading to degeneration of the nerve tissue.
An outbreak of the disease was reported in 2009. To protect people from new infections, the United States and Canada have banned the production, importation, and prescription of medicines with animal components that can transmit the prion protein. The growth hormone derived from the pituitary gland and products based on animal blood serum were also included in this list.
However, Actovegin producer claims that the technology of Actovegin production excludes the presence of protein components with antigenic and pyrogenic properties. It is known that the size of prion particles is approximately 35-36 kDa. The technological process of Actovegin production does not allow particles larger than 5 kDa to enter the final product.
According to this statement document posted on Actovegin official website the biological safety of the raw material known as Actovegin® concentrate was confirmed by the Certificate of Conformity of the European Directorate for the Quality of Medicines (EDQM) No. R1-CEP 2004-235-Rev 00 dated 04.12 2009. Furthermore, it was established that the production of Actovegin® meets all requirements for the prevention of spongiform encephalopathy stipulated by the European Medicines Agency (EMA) document EMA/410/01 rev.3 “Note for guidance on minimizing the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products”.
The manufacturer points out that so far no cases of Creutzfeldt-Jakob disease have been reported in any country where Actovegin is used in connection with the use of the drug.
Currently, Actovegin keeps being universally used for the treatment of cerebral vascular diseases and cognitive disorders of varying severity.
Anecdotal Actovegin Reddit reviews
There are several discussions on Reddit about Actovegin effects on various health aspects. Read whether Actovegin can help with brain fog and other Actovegin Reddit discussion threads. There is also a heated Reddit discussion of Actovegin uses in big sports.
Along with anecdotal experiences a group of scientists from Cardiff University in the UK shared their study on Actovegin in terms of common sport-related injuries. In their small pilot study, they came to the conclusion that “players in the Actovegin treatment group were able to return to play 8 days earlier (95% CI -1.249 to -14.7510) compared to physiotherapy alone (p=0.033). No adverse reactions were recorded in any of the participants.”
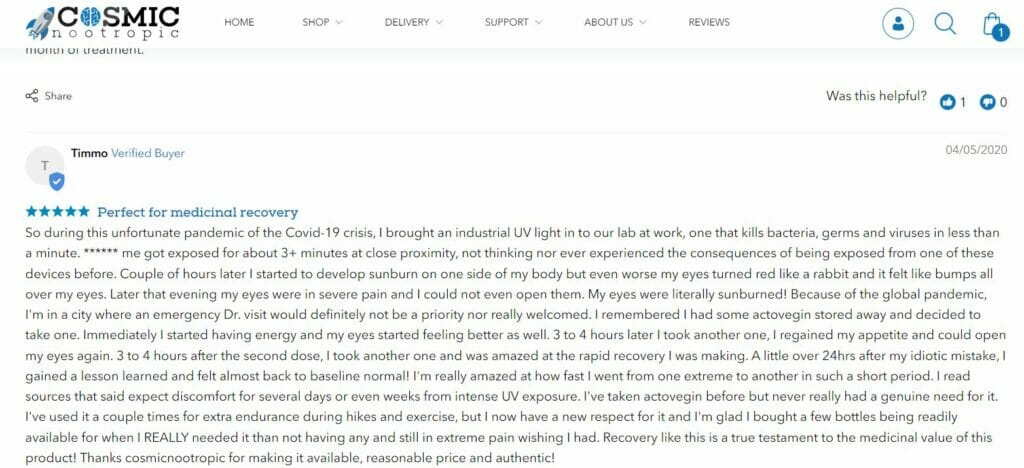
You can also familiarize yourself with Actovegin reviews from customers at CosmicNootropic.
Where to buy Actovegin?
Actovegin legal status is unclear in many parts of the world. The nootropic cannot be bought OTC in the USA, Canada, or the UK. However, the drug is used for medicinal purposes at the discretion of general practitioners in some European countries, in Russia and CIS countries, in China, and in South Korea.
You can buy Actovegin online at CosmicNootropic. CosmicNootropic offers Actovegin 5ml x 5 ampoules and Actovegin 200mg x 50 pills with fast US delivery and worldwide shipping! Always be sure to do your research before purchasing any nootropic supplement to ensure you are getting a quality product.
What is Actovegin dosage?
Actovegin 200 mg tablets
Orally, without chewing, before meals, with a small amount of liquid.
- Peripheral circulation disorders and their consequences. 1-2 tablets 3 times a day (600 – 1200 mg/day). Duration of treatment from 4 to 6 weeks.
- Dementia. 2 tablets 3 times a day (1200 mg/day). Overall duration of treatment is 20 weeks.
- Post-stroke cognitive disorders. In the acute period of ischemic stroke, starting from day 5-7, 2000 mg per day intravenously, up to 20 infusions. After the course, cycling Actovegin is recommended with a transition to tablet form, 2 tablets 3 times a day (1200 mg/day). Overall duration of treatment is 6 months.
- Diabetic polyneuropathy. 2000 mg per day i.v., 20 infusions with transition to tablet form 3 tablets 3 times a day (1800 mg/day) with duration from 4 to 5 months.
Actovegin 5 ml ampoules
According to the instructions Actovegin solution can be taken intravenously, or intramuscularly. The drug can also be added to solutions for infusion.
For infusion administration 10 to 50 ml of the drug should be added to 200-300 ml of basic solution (isotonic sodium chloride solution or 5% glucose solution). The infusion rate is about 2 ml/min.
For intramuscular injections use no more than 5 ml of the drug, which is to be injected slowly, because the solution is hypertonic. Depending on the severity of the clinical picture, initially 10-20 ml of the drug should be administered intravenously or intra-arterially, daily. For further treatment, 5 ml intravenously or intramuscularly slowly, daily or several times a week.
- Post-stroke cognitive disorders. In the acute period of ischemic stroke, starting from day 5-7, 2000 mg per day intravenously, up to 20 infusions. After the course, cycling Actovegin is recommended with a transition to tablet form, 2 tablets 3 times a day (1200 mg/day). Overall duration of treatment is 6 months.
- Dementia. 2000 mg per day intravenously. Overall duration of treatment is four weeks.
- Peripheral circulation disorders and their consequences. 800 – 2000 mg/day intravenously or intra-arterially. Duration of treatment 4 weeks.
- Diabetic polyneuropathy. 2000 mg per day i.v., 20 infusions with transition to tablet form 3 tablets 3 times a day (1800 mg/day) with duration from 4 to 5 months.
Read: How are athletes taking Actovegin?
Actovegin injections vs Actovegin pills, which one is better?
Generally speaking, Actovegin injections are more potent compared with the pill form. This is due to the fact that the pharmaceutical directly enters the blood flow bypassing the gastrointestinal tract. The onset of action arrives faster. Hence Actovegin injections are mainly used in acute conditions such as trauma or injury. The course of treatment is also shorter than with Actovegin pills.
However, Actovegin pills have their advantages too. The main one is the convenience of use. Pill administration does not require help from a medical professional, which might be requested for IM or IV injection. Pills tend to have more prolonged action since the digestive process delays the absorption of the substance, resulting in slower action of Actovegin tablets. This may be particularly useful for patients suffering from chronic illnesses.
As we can see each form of the drug has its own pros and cons and is used in different cases. When making a choice, it is necessary to take into account the state of health and the presence of pathologies too. The correct form of the drug can only be prescribed by the attending physician depending on the patient’s disease.
Conclusion
To sum it up Actovegin nootropic, antihypoxic and antioxidant action can be used in a wide range of neurological diseases of the central and peripheral nervous system, that mainly originate from hypoxia, ischemia, and oxidative stress. The drug can be used in vascular diseases of the brain, and in cognitive disorders caused by vascular or vascular-degenerative factors.
Positive Actovegin reviews and numerous studies published in local scientific journals vouch for its effectiveness, which is yet to be confirmed by more large-scale studies of its effects and safety.
The material provided in this article cannot be viewed as a substitute for a visit to the doctor. Before you start taking any particular drug, be sure to consult with a proper specialist.
Actovegin review bibliography
- Saletu B, Grunberger J, Linzmayer L et al (1990). EEG brain mapping and psychometry in age–associated memory impairment after acute and 2–week infusions with the hemoderivative Actovegin: double–blind, placebo–controlled trials. https://pubmed.ncbi.nlm.nih.gov/2135068/
- Semlitsch HV, Anderer P, Saletu B et al (1990). Topographic mapping of cognitive event-related potentials in a double-blind, placebo-controlled study with the hemoderivative Actovegin in age–associated memory impairment. https://pubmed.ncbi.nlm.nih.gov/2132641/
- Jacob S, Dietze GJ, Machicao F et al (1996). Improvement of glucose metabolism in patients with type II diabetes after treatment with hemodialysate. https://pubmed.ncbi.nlm.nih.gov/8901147/
- Jansen V, Brukner GV (2002). Treatment of chronic cerebrovascular insufficiency using Actovegin forte dragees (double-blind, placebo-controlled study). https://www-rmj-ru.translate.goog/articles/obshchie-stati/Lechenie_hronicheskoy_cerebrovaskulyarnoy_nedostatochnosti_s_ispolyzovaniem_draghe_Aktovegin_forte_dvoynoe_slepoe_placebo-kontroliruemoe_issledovanie/?_x_tr_sl=ru&_x_tr_tl=en&_x_tr_hl=ru&_x_tr_pto=wapp
- Jansen W, Beck E (2002). Treatment of diabetic polyneuropathy. Controlled double blind study. https://medgate-ru.translate.goog/article/110/118056/?_x_tr_sl=ru&_x_tr_tl=en&_x_tr_hl=ru&_x_tr_pto=wapp
- Sych YuP, Zilov AV (2003). Possibilities of using actovegin in the treatment of diabetes mellitus. https://www-probl–endojournals-ru.translate.goog/jour/article/view/11635?_x_tr_sl=ru&_x_tr_tl=en&_x_tr_hl=ru&_x_tr_pto=wapp
- Kunts H, Shumann H (2004). Actovegin use in moderate dementia: results of a multicenter double blind placebo-controlled randomized study. https://elibrary.ru/item.asp?id=17248053
- Strokov IA et al (2006). Therapeutic correction of diabetic polyneuropathy and encephalopathy with Actovegin. https://www-rmj-ru.translate.goog/articles/obshchie-stati/Terapevticheskaya_korrekciya_diabeticheskoy_polinevropatii_i_encefalopatii_Aktoveginom/?_x_tr_sl=ru&_x_tr_tl=en&_x_tr_hl=ru&_x_tr_pto=wapp
- Shishkova VN (2007). Prospects for the use of Actovegin in patients with metabolic syndrome and prediabetes. Modern concepts of carbohydrate metabolism disorders. https://www-rmj-ru.translate.goog/articles/endokrinologiya/Perspektivy_primeneniya_preparata_Aktovegin_u_pacientov_s_metabolicheskim_sindromom_i_prediabetom_Sovremennye_predstavleniya_o_narusheniyah_uglevodnogo_obmena/?_x_tr_sl=ru&_x_tr_tl=en&_x_tr_hl=ru&_x_tr_pto=wapp
- Ziegler D, Movsesyan L, Mankovsky B et al (2009). Treatment of symptomatic polyneuropathy with Actovegin in Type 2 diabetic patients. https://diabetesjournals.org/care/article/32/8/1479/38975/Treatment-of-Symptomatic-Polyneuropathy-With?searchresult=1
- Elmlinger MW, Kriebel M, Ziegler D (2011) Neuroprotective and anti-oxidative effects of the hemodialysate Actovegin on primary rat neurons in vitro. https://pubmed.ncbi.nlm.nih.gov/21983748/.
- Lee P, Rattenberry A, Connelly S, Nokes L (2011). Our experience on Actovegin, is it cutting edge? https://pubmed.ncbi.nlm.nih.gov/21271496/
- Machicao F, Muresanu DF, Hundsburger H et al (2012). Pleiotropic neuroprotective and metabolic effects of Actovegin’s mode of action. https://pubmed.ncbi.nlm.nih.gov/22910148/
- Dieckmann A, Kriebel M, Andriambeloson E et al (2012). Treatment with Actovegin improves sensory nerve function and pathology in streptozotocin-diabetic rats via mechanisms involving inhibition of PARP activation. https://pubmed.ncbi.nlm.nih.gov/22020669/
- Kozlov PV, Ivannikov YuN, Bagaeva II (2012). Prevention of perinatal pathology in the syndrome of growth retardation of premature fetus. https://cyberleninka.ru/article/n/profilaktika-perinatalnoy-patologii-pri-sindrome-zaderzhki-rosta-nedonoshennogo-ploda
- Guekht A, Skoog I et al (2017). ARTEMIDA Trial (A Randomized Trial of Efficacy, 12 Months International Double-Blind Actovegin): A Randomized Controlled Trial to Assess the Efficacy of Actovegin in Poststroke Cognitive Impairment. https://pubmed.ncbi.nlm.nih.gov/28432265/
- Suchkov IA, Mzhavanadze ND et al (2022). Efficacy and safety of Actovegin in the treatment of intermittent claudication: results of an international, multicenter, placebo-controlled, randomized, phase IIIb clinical trial (APOLLO). https://pubmed.ncbi.nlm.nih.gov/36264097/

